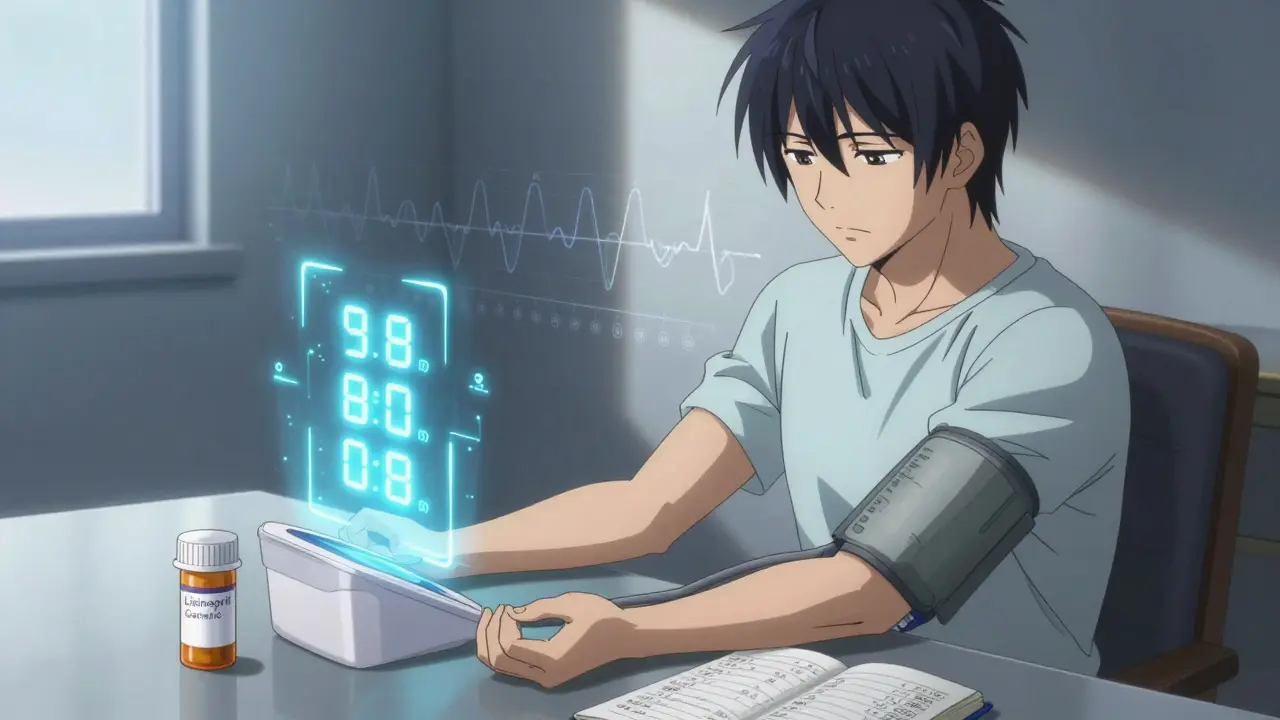
Switching from a brand-name drug to a generic version is one of the most common changes in modern healthcare. In fact, generics make up over 90% of all prescriptions filled in the U.S., and that number is growing. The reason is simple: they save money-often 80% less than the brand version. But just because they’re cheaper doesn’t mean you should ignore what happens after you make the switch. Your body might react differently, even if the science says it shouldn’t. Here’s how to watch for signs that the switch isn’t working as expected-and what to do next.
Why Generics Are Supposed to Work the Same
Generic drugs aren’t knockoffs. They’re required by the FDA to contain the exact same active ingredient, in the same strength, and delivered the same way as the brand-name drug. That means if you’re taking lisinopril for high blood pressure, your generic version has the same molecule doing the same job. The FDA demands proof through bioequivalence studies: the generic must deliver the drug into your bloodstream at the same rate and amount as the original, within a strict 80-125% range. This isn’t a guess-it’s measured in labs using blood samples and statistical models.Manufacturers also have to prove their generics stay stable for at least a year, sometimes two. That’s the same shelf life as the brand. The FDA inspects factories overseas and in the U.S. more than 1,100 times a year. And since 2022, generic makers pay nearly half a billion dollars annually in fees to fund this oversight. So yes, the system is strict. But strict doesn’t mean perfect.
When Things Don’t Go as Planned
Most people-over 90%-switch to generics with no issues at all. But for a small group, things change. You might notice your blood pressure isn’t as controlled, your seizures are coming back, or your thyroid symptoms are returning. These aren’t imagined. They’re real, and they happen more often with certain types of drugs.Drugs with a narrow therapeutic index are the biggest concern. That means there’s a tiny window between the dose that works and the dose that causes harm. Small changes in how the drug is absorbed can tip you out of that window. These include:
- Warfarin (blood thinner)
- Levothyroxine (thyroid hormone)
- Lamotrigine and phenytoin (anti-seizure meds)
- Cyclosporine and tacrolimus (organ transplant drugs)
- Some antidepressants like bupropion
A Harvard study published in JAMA Internal Medicine found that patients on these drugs were more likely to experience problems after switching. One woman in Sydney switched from brand-name levothyroxine to a generic and noticed her fatigue worsened, her heart started racing, and her TSH levels jumped from 2.1 to 7.8 within three weeks. Her doctor didn’t think it was the medication-until she showed the lab results. She switched back, and her numbers normalized in two weeks.
What to Monitor After the Switch
You don’t need to panic. But you do need to pay attention. Here’s what to track, depending on your condition:- High blood pressure: Check your readings at home twice a day for the first two weeks. Write them down. If your average systolic number jumps by more than 10 points, call your doctor.
- Diabetes: Track your fasting blood sugar and HbA1c. Get a blood test at 4 and 8 weeks after switching. A rise of 0.5% or more on HbA1c is a red flag.
- Thyroid: Get your TSH and free T4 tested at 4 weeks. Levothyroxine is especially sensitive to small changes in formulation. Even a 10% difference in absorption can throw you off.
- Epilepsy or seizures: Keep a seizure diary. Note frequency, duration, and severity. If you have more than one seizure in two weeks that you didn’t have before, seek help immediately.
- Depression or anxiety: Track your mood daily using a simple 1-10 scale. If your average score drops by 2 points or more over 10 days, talk to your prescriber.
Consumer Reports found that 24% of people who switched to generics started monitoring their health more closely. The top metrics? Blood pressure (38%), blood sugar (29%), and seizure frequency (17%). That’s not paranoia-that’s smart.

Check the Pill, Check the Code
Not all generics are the same. Different manufacturers use different inactive ingredients-fillers, dyes, binders. These don’t affect the active drug, but they can change how fast it dissolves in your stomach. That’s why two generics for the same drug might behave differently.Always check the National Drug Code (NDC) on the bottle. It’s a 10-digit number. If it changes from your last prescription, that’s a new generic maker. Don’t assume it’s the same. Ask your pharmacist: “Is this the same manufacturer as last time?” If they say no, ask if you can stick with the one that worked before.
The FDA’s Orange Book lists which generics are rated as therapeutically equivalent. But it doesn’t tell you which manufacturer makes which version. That’s up to the pharmacy. So if you’re on a narrow-therapeutic-index drug, ask for the same brand of generic every time. You have that right.
When to Call Your Doctor
You don’t need to wait for a crisis. If you notice any of these, reach out within a week:- New or worsening side effects (rash, dizziness, nausea, mood changes)
- Loss of symptom control (higher blood pressure, more seizures, fatigue returning)
- Pill looks different-color, shape, markings-and you didn’t expect it
- You feel “off” but can’t explain why
Don’t just assume it’s stress or aging. Your body might be telling you the medication isn’t working the same way. Your doctor can order tests, switch you back, or try another generic. There’s no shame in that.

Reporting Problems Helps Everyone
If you have a bad experience, report it. The FDA’s MedWatch program collects reports from patients and doctors. In 2022, they got over 1.2 million reports-but only 15% were about generic drugs. That means most people don’t report. But every report matters.When you report, include:
- The exact name of the drug (generic and brand, if you know it)
- The NDC number and lot number from the bottle
- When you started the new version
- What symptoms you noticed and when
- Any lab results or doctor visits
The FDA promises to acknowledge your report within 15 business days and investigate serious cases within 30 days. Your report could help catch a bad batch before it affects someone else.
What the Data Really Shows
Let’s be clear: generics are safe. The FDA, WHO, and major medical groups all agree. Studies show no overall difference in effectiveness between brand and generic drugs across millions of patients. But data doesn’t always capture individual variation. A 92% success rate still means 8 out of 100 people might have trouble. That’s not a small number-it’s 80,000 people in Australia alone.PatientsLikeMe tracked 42,000 people who switched to generics. 92.7% had no change. But 7.3% had some variation. And 1.2% needed medical help. That’s not a failure of generics-it’s a reminder that medicine isn’t one-size-fits-all. Your body is unique. Your response matters.
Bottom Line: Stay Aware, Not Afraid
Switching to a generic is smart. It saves money, reduces waste, and keeps healthcare affordable. But don’t treat it like a set-it-and-forget-it decision. Monitor your body. Track your numbers. Know your pills. Speak up if something feels off.Generics work for most people, most of the time. But if you’re on a drug where small changes matter-thyroid, epilepsy, heart meds-don’t skip the follow-up. Your health isn’t a statistic. It’s your life. And you’re the best person to notice when something’s wrong.


Diksha Srivastava
January 31, 2026 AT 09:37Love this post! I switched my mom to generic levothyroxine last year and she was fine at first, but then she started feeling wiped out all the time. We didn’t think much of it until her TSH jumped. Got her back on the brand, and boom-energy returned in a week. Never assume generics are always identical. Your body knows.
Stay alert, not scared. You got this!
Sidhanth SY
February 2, 2026 AT 00:58Honestly? I’ve been on generic warfarin for 3 years now. No issues. But I check my INR every 2 weeks like clockwork. That’s the key-monitoring. Doesn’t matter if it’s brand or generic, if you’re on something with a narrow window, you gotta stay on top of it.
Also, pharmacies love to swap generics without telling you. Always check the NDC. I keep a screenshot of mine in my phone.
Adarsh Uttral
February 3, 2026 AT 10:05bro i switched to generic lamotrigine last year and my seizures got worse like 3x in 2 weeks
doc said it was stress lol
i showed him the pill looked different and he finally listened
switched back and now im chill af
tl;dr-pills change, you gotta notice
Shubham Dixit
February 4, 2026 AT 00:26Why are we even having this conversation? In India, we’ve been using generics for decades and the system works. The FDA is overregulated, and Americans act like every pill is a lottery ticket. If your body reacts badly, maybe you’re just weak. We don’t whine about generics in developing countries-we take what works and move on.
Stop treating medicine like a luxury product. It’s not your personal experiment. It’s public health.
KATHRYN JOHNSON
February 5, 2026 AT 00:08As a clinical pharmacist with 22 years of experience, I must emphasize that bioequivalence standards are rigorously enforced. The notion that generics vary significantly in efficacy is a myth perpetuated by anecdotal reports and pharmaceutical marketing. The 1.2% adverse event rate cited is statistically insignificant when compared to the millions of patients successfully transitioned annually. Your concern, while well-intentioned, lacks empirical grounding.
Sazzy De
February 5, 2026 AT 11:52I switched my dad to generic cyclosporine after his transplant and he was fine for months then started feeling off
we didn’t think much of it until his kidney numbers dipped
turned out the new batch had a different filler
pharmacist didn’t even tell us it changed
now we only take the one with the blue capsule
just check the label
Carolyn Whitehead
February 6, 2026 AT 22:15My sister’s on generic bupropion and she said she felt like a zombie for two weeks
she thought it was just postpartum stuff
then she switched back to the brand and suddenly she was laughing again
don’t ignore how you feel
your mood matters more than the price tag
Amy Insalaco
February 7, 2026 AT 22:36While the FDA’s bioequivalence paradigm is statistically sound, it fundamentally fails to account for pharmacokinetic heterogeneity at the individual level, particularly with regard to first-pass metabolism polymorphisms, gut microbiome variability, and transporter protein expression profiles. The 80-125% confidence interval is an aggregate metric that obscures clinically significant inter-individual variance, especially in patients with polypharmacy, hepatic impairment, or altered gastric pH. The reliance on population-based bioequivalence thresholds as a proxy for therapeutic equivalence is a reductionist fallacy that risks iatrogenic harm in vulnerable subpopulations.
Marc Bains
February 8, 2026 AT 14:18As a Nigerian immigrant in the U.S., I’ve seen both systems. In Lagos, generics are the only option-and they save lives. But here, people treat them like they’re suspect. It’s not about the pill-it’s about trust. Talk to your pharmacist. Ask for the same manufacturer. Track your numbers. Don’t let fear or arrogance stop you from being your own advocate.
Medicine is global. Your health shouldn’t be a privilege.
Rob Webber
February 9, 2026 AT 02:01So now we’re treating patients like lab rats? You’re telling me I have to keep a seizure diary, check my blood pressure twice a day, memorize NDC codes, and file FDA reports just to get my damn medication to work? This isn’t healthcare-it’s a full-time job. And who has time for that? My boss doesn’t care if my TSH is off.
Someone needs to fix the system, not make patients its unpaid interns.
Lisa McCluskey
February 10, 2026 AT 03:43I’ve worked in pharmacy for 18 years. The biggest issue isn’t the generic-it’s the switch without communication. Pharmacies rotate suppliers to save costs. Patients don’t know. Doctors don’t know. So you get a new pill, no warning, and then symptoms show up.
Ask for the same generic every time. Write it on the prescription. Simple. No drama. Just clarity.
owori patrick
February 10, 2026 AT 08:11Back home in Nigeria, we don’t even have brand-name drugs most times. We use generics and survive. But I agree with the post-you need to watch your body. I had a cousin on generic levothyroxine, she got dizzy, lost weight, then we found out the batch was faulty. We reported it. They pulled it. Now we know to check the lot number.
It’s not about where you’re from. It’s about paying attention.
Claire Wiltshire
February 10, 2026 AT 12:29This is an excellent, well-researched guide. Thank you for highlighting the importance of patient monitoring and the role of inactive ingredients. The FDA’s Orange Book is a valuable resource, but its limitations are often misunderstood. Pharmacists should be encouraged to provide patients with manufacturer-specific information when dispensing narrow-therapeutic-index drugs. A simple note on the prescription label-‘Do not substitute’-can prevent serious adverse events. Your emphasis on reporting to MedWatch is critical. Every data point matters.