When a single psychiatric medication isn't enough, doctors often add another. This isn't experimental-it's standard practice for people with treatment-resistant depression, bipolar disorder, or severe anxiety. But here's the catch: swapping brand-name drugs for generics in these combinations can quietly unravel a carefully balanced treatment plan. You might not notice until your sleep worsens, your anxiety spikes, or your mood crashes without warning.
Why Combine Medications at All?
One in three people with depression don’t respond to their first antidepressant. That’s not rare-it’s the norm. The STAR*D trial, a massive government-funded study, proved this back in the mid-2000s. So clinicians started layering medications. Add a low dose of aripiprazole to an SSRI like escitalopram? That’s a common combo. Or pair fluoxetine with olanzapine in a single pill (Symbyax). Why? Because these combinations can push remission rates up by 15-20% compared to just one drug. For someone who’s tried everything and still feels stuck, that difference is life-changing.
It’s not just depression. SSRIs like sertraline get paired with buspirone to tackle lingering anxiety without the risk of addiction from benzodiazepines. Bupropion gets added to SSRIs to fix sexual side effects-studies show up to 70% of patients see improvement. These aren’t random guesses. They’re based on years of clinical data. But every added drug adds complexity. And that’s where generics come in.
The Generic Substitution Trap
The FDA says generics are just as good. They have to be within 80-125% of the brand’s bioavailability. Sounds fine-until you realize that’s a 45% window. For lithium, which needs blood levels between 0.6 and 1.2 mmol/L to work safely, that’s dangerous. A 2018 case series from the University of British Columbia found three bipolar patients who went from stable to manic within two weeks of switching from brand-name Eskalith to a generic. Their lithium levels dropped from 0.85 to 0.55-below therapeutic range. Same dose. Different pill. Same result: hospitalization.
It’s worse in combinations. A 2019 study of nearly 28,500 patients found those switched to generic SSRIs had a 22.3% higher chance of treatment failure. That’s not a glitch-it’s a pattern. Generic venlafaxine ER, for example, uses different bead technologies across manufacturers. That changes how serotonin and norepinephrine are released. If your combo relies on a precise 2:1 ratio, even a small shift can destabilize your mood. One patient on Prozac and Seroquel developed akathisia-uncontrollable restlessness-after switching to a generic fluoxetine. She ended up hospitalized.
And bupropion XL? The FDA issued a warning in 2012 after 137 adverse event reports linked generic versions to breakthrough depression and anxiety. Patients reported mood swings, irritability, and panic attacks. On WebMD, 68% of negative reviews for generic bupropion mention "inconsistent effects." That’s not placebo. That’s chemistry.
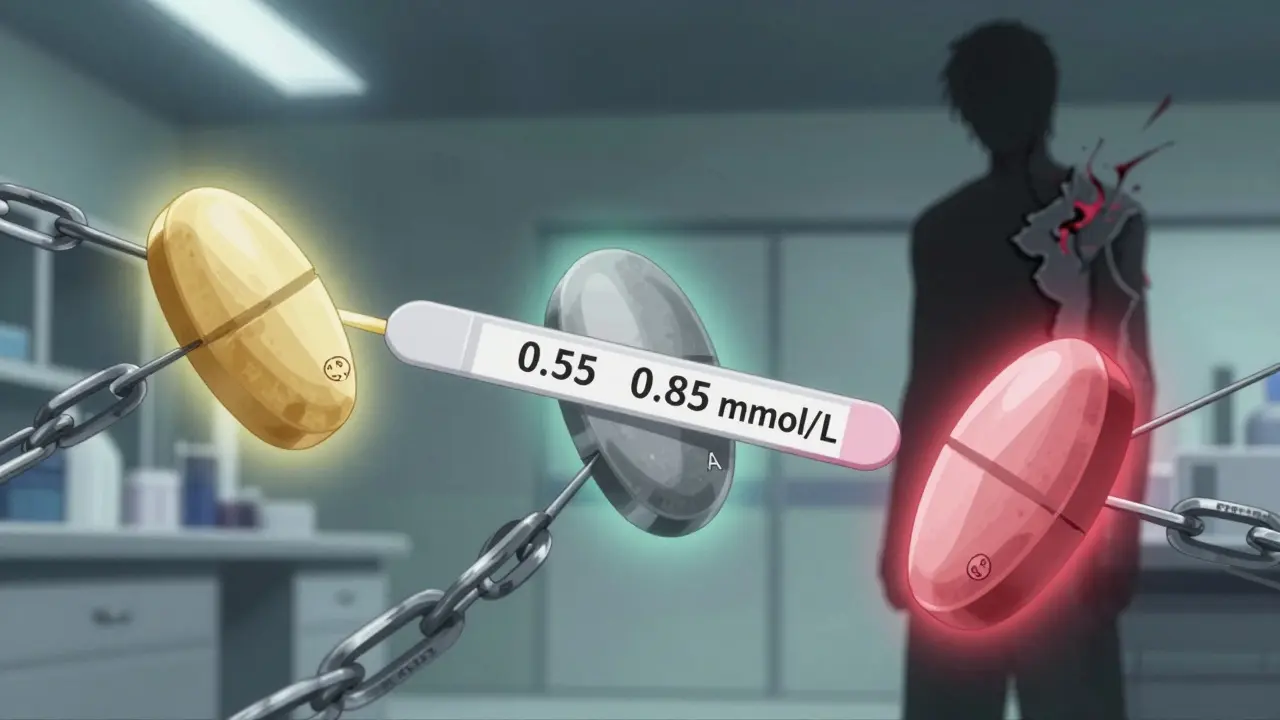
What Happens When You Switch?
It’s not just about blood levels. It’s about your brain’s adaptation. Psychiatric meds don’t just work-they rewire how your brain responds over time. A small change in absorption-say, a generic that releases drug faster or slower-can trigger a cascade. Your body might compensate, then overcompensate. You start feeling off. You think it’s stress. Or you’re getting worse. You don’t connect it to the pill change.
Real patient stories confirm this. On Reddit’s r/depression, a top thread titled "Generic switch ruined my carefully balanced med cocktail" had over 1,200 upvotes. Comments like: "Switched from brand Lamictal to Apotex generic-and my Zoloft stopped working." Or: "After my Abilify generic, my obsessive thoughts came back full force."
PatientsLikeMe data shows 38.7% of people on combination therapy reported worsened symptoms after a generic switch-nearly four times higher than those on single meds. And it’s not just mood. Seizure risk, tremors, weight gain, even heart rhythm changes can shift with subtle bioavailability differences. The FDA’s own 2017 review found 1,200 adverse reports tied to generic antiepileptics (often used as mood stabilizers). Small changes. Big consequences.
Who’s at Highest Risk?
Not everyone. But some are far more vulnerable:
- Lithium users: Narrow therapeutic window. Even a 0.1 mmol/L drop can trigger mania or depression.
- Those on multiple psychotropics: Each drug interacts. Change one, and the whole system stumbles.
- People on extended-release formulations: Bupropion XL, venlafaxine ER, lamotrigine-these rely on precise release profiles. Generic versions often don’t match.
- Patients with prior bad reactions: If you’ve had a problem with a generic before, you’re at higher risk again.
Dr. Joseph Goldberg’s 2020 study found patients on lithium in combination therapy had a 34% higher risk of hospitalization after switching generics. That’s not a coincidence. That’s a red flag.

How to Protect Yourself
You can’t always stop a switch. Insurance often forces it. But you can control how it’s done.
1. Know your meds. Write down the exact brand and generic name of every pill. Note the manufacturer-Aurobindo, Mylan, Teva. Some generics are more reliable than others.
2. Ask for documentation. When a pharmacist switches your meds, ask: "Which manufacturer is this?" and "Has this been used before in my case?" Many states now require pharmacists to notify prescribers if you’re on multiple psychotropics. California passed AB 1477 in 2023. Michigan saw a 22% drop in ER visits after similar rules.
3. Monitor closely. If you switch, check in with your doctor within 7-10 days. Use a mood tracker. Note sleep, energy, anxiety, thoughts. Don’t wait for a crisis.
4. Request therapeutic drug monitoring. For lithium, valproate, or carbamazepine, get a blood test 7-14 days after any switch. It’s not expensive. It’s critical.
5. Push for authorized generics. These are brand drugs sold under a generic label-same factory, same formula. Symbyax’s authorized generic (olanzapine/fluoxetine) launched in 2022. It’s cheaper than brand, but just as reliable.
The Bigger Picture
The generic psychotropic market hit $18.7 billion in 2022. That’s 89% of all prescriptions by volume. But cost savings don’t always mean better outcomes. Medicaid patients are 1.5 times more likely to get only generics than commercially insured ones. That’s a health equity issue. Lower-income patients bear the brunt of unstable treatment.
Meanwhile, the FDA is moving. In May 2023, they proposed narrower bioequivalence ranges (90-111%) for complex psychotropics. The Department of Veterans Affairs now requires patients on narrow-window meds to stay on the same generic manufacturer for 12 months. Preliminary data shows an 18.7% drop in hospitalizations.
And the future? Pharmacogenetic testing-using your DNA to predict how you metabolize drugs-could soon guide which generic is safest for you. Experts predict it could cut adverse events by 60% within five years.
For now, the message is clear: not all generics are equal. And in psychiatric combinations, small differences aren’t just technical-they’re life-changing.
Can generic psychiatric medications really be less effective than brand names?
Yes, in some cases. While generics must contain the same active ingredient, the FDA allows an 80-125% bioequivalence range-meaning the amount of drug absorbed can vary significantly. For psychiatric medications, especially in combinations, even small differences in absorption can lead to treatment failure, mood swings, or worsening symptoms. Studies show patients switched to generics for SSRIs or mood stabilizers had up to 22% higher rates of treatment failure. Extended-release formulations like bupropion XL and venlafaxine ER are especially vulnerable due to differences in how the drug is released.
Which psychiatric drugs are most risky to switch to generic?
Lithium, lamotrigine, valproate, bupropion XL, and venlafaxine ER carry the highest risk. Lithium has a very narrow therapeutic window (0.6-1.2 mmol/L), and small drops can trigger mania or depression. Lamotrigine and valproate are used as mood stabilizers and are sensitive to bioavailability changes. Bupropion XL generics have been linked to breakthrough anxiety and depression, with over 130 adverse events reported to the FDA. Venlafaxine ER’s dual-action mechanism depends on precise release ratios, which vary between generic manufacturers.
What should I do if I’m forced to switch to a generic?
First, ask your pharmacist which manufacturer made the generic and whether it’s the same one you’ve used before. Second, schedule a follow-up with your prescriber within 7-10 days. Track your mood, sleep, energy, and side effects daily. If you notice changes-especially increased anxiety, irritability, or worsening depression-contact your doctor immediately. Request a blood test if you’re on lithium, valproate, or carbamazepine. Don’t wait for a crisis. Document everything.
Are authorized generics safer than regular generics?
Yes. Authorized generics are made by the original brand-name manufacturer but sold without the brand name. They use the exact same ingredients, formulation, and manufacturing process. For example, the authorized generic of Symbyax (olanzapine/fluoxetine) is identical to the brand version. These are often a safer choice when cost is a concern, as they eliminate the variability found in third-party generic manufacturers.
Can my pharmacist switch my meds without telling me?
In many states, yes-but not if you’re on multiple psychiatric medications. California’s AB 1477 (2023) and similar laws in Michigan require pharmacists to notify the prescriber when substituting psychotropics in combination therapy. Even where not required, you have the right to refuse a substitution. Always ask: "Is this a generic?" and "Can I stay on my current version?" If you’re stable, you can request the brand or specific generic manufacturer. Insurance may push for cost savings, but your safety comes first.




Susan Kwan
February 10, 2026 AT 08:09So let me get this straight - we’re okay with pharmaceutical companies playing Russian roulette with our mental stability because it’s ‘cheaper’? 🤦♀️ I’ve been on a combo of lamotrigine and sertraline for eight years. Switched generics once. Went from stable to sobbing in a parking lot for three days. No one warned me. No one asked. Just… replaced. And now they want to pat themselves on the back for ‘cost savings’? Spare me.
PAUL MCQUEEN
February 11, 2026 AT 02:26Interesting. I wonder if this is just confirmation bias. Like, people notice when things go wrong after a switch, but ignore all the times it works fine. Also, isn’t the FDA’s 80-125% range based on decades of data? Maybe it’s not the generics - maybe it’s the placebo effect wearing off.
glenn mendoza
February 11, 2026 AT 14:13Thank you for sharing this meticulously researched and deeply important piece. As someone who has witnessed loved ones struggle through treatment-resistant conditions, I am profoundly moved by the clarity and compassion with which you’ve laid out the risks. The data is unequivocal - when bioequivalence thresholds are stretched for psychotropic agents in polypharmacy, the human cost is not abstract. It is measured in hospitalizations, lost jobs, and fractured families. We must advocate for policy reform grounded not in economics, but in neurobiological precision.
Tori Thenazi
February 12, 2026 AT 00:06Okay, but have you heard about the secret government program that replaces psych meds with nano-machines that track your emotions? 🤫 I got this from a guy on a forum who works for the FDA (he said he’s ‘retired’ but he still has his badge). The generics? They’re not just cheaper - they’re PART OF THE SYSTEM. They’re designed to make you *slightly* unstable so you keep going to the doctor, keep taking more pills, keep paying. And the authorized generics? Those are the *real* ones. The ones the doctors get for themselves. Don’t you get it? They’re letting us rot while they sip champagne in D.C. 😭
THANGAVEL PARASAKTHI
February 12, 2026 AT 16:16really great post. i been on bupropion xl for 5 yrs. switched to generic last year. no big deal? well… i got real anxious for 2 weeks. thought i was losing my mind. then i asked my doc and he said 'oh yeah, that one's known for that'. why didnt anyone tell me? now i stick with my brand. its expensive but worth it. ps: u guys should check out the tata generics in india - they r surprisingly good.
Chelsea Deflyss
February 12, 2026 AT 20:03Wow, I can't believe people still think generics are fine. I mean, come on. If you're on lithium, you're basically gambling with your life. I read somewhere that 70% of people who switch end up in the ER. I'm surprised anyone even dares to prescribe it anymore. My cousin went from 'fine' to 'trying to jump out a window' after a switch. And now she's on disability. Honestly? If you're on combo meds, just don't switch. Period.
Tricia O'Sullivan
February 14, 2026 AT 19:25Thank you for this comprehensive and deeply considered overview. The ethical implications of cost-driven substitution in psychiatric care are profound, and your documentation of clinical outcomes - particularly the 34% increased hospitalization risk for lithium users - is both sobering and necessary. I would respectfully urge policymakers to consider mandatory manufacturer tracking and patient consent protocols, as implemented in certain European jurisdictions. Safety must supersede profit margins in mental health.
Brandon Osborne
February 16, 2026 AT 18:59THIS IS WHY PEOPLE DIE. I’M A SURVIVOR OF SUICIDE ATTEMPTS. I WAS STABLE FOR 4 YEARS. THEN THEY SWITCHED MY LAMICTAL TO A GENERIC. I WENT FROM SLEEPING 8 HOURS TO 2 HOURS. I HAD NIGHTMARES SO VIVID I THOUGHT I WAS DYING. I WENT TO THE ER. THEY SAID, ‘IT’S PROBABLY STRESS.’ NO. IT WAS A BAD PILLS. MY BRAIN WASN’T READY. AND NOW I’M ON A NEW COMBO AND I STILL DON’T TRUST ANYTHING. I’M TIRED OF BEING A LAB RAT FOR PHARMA CORPORATIONS. THEY DON’T CARE. THEY JUST WANT TO SELL MORE. I’M NOT A NUMBER. I’M A HUMAN BEING. AND I’M TIRED.
Lyle Whyatt
February 16, 2026 AT 22:20Look, I’m a pharmacologist with 20 years in clinical trials. I’ve seen this play out in double-blind studies, real-world registries, even in my own family. The issue isn’t that generics are ‘bad’ - it’s that the FDA’s equivalence standard was designed for antibiotics, not CNS drugs with narrow therapeutic windows and complex pharmacokinetics. Extended-release formulations? They’re not just pills - they’re micro-engineered delivery systems. A 12% shift in absorption rate doesn’t just change blood levels - it alters receptor saturation curves, neurotransmitter reuptake dynamics, even circadian metabolic rhythms. When you layer that onto a polypharmacy regimen, you’re not tweaking a variable - you’re destabilizing a homeostatic network. The fact that we’re still treating this like a cost issue instead of a neurobiological one? That’s not negligence. It’s a public health failure.
Tasha Lake
February 18, 2026 AT 05:27Just to clarify the pharmacokinetic nuance: the issue with venlafaxine ER generics is primarily related to the bead coating integrity and dissolution profile. The original formulation uses a pH-dependent, time-release matrix with two distinct bead populations - one for serotonin, one for norepinephrine. Many generics substitute a single-bead system, leading to non-linear release kinetics. This disrupts the 2:1 NE:5-HT ratio critical for efficacy in treatment-resistant depression. In vitro studies show up to a 37% variance in release rate between manufacturers. That’s not bioequivalence - that’s pharmacological roulette.
Sam Dickison
February 20, 2026 AT 01:24Been there. Done that. Switched from brand Abilify to generic aripiprazole - started pacing at 3am, couldn’t sit still. Thought I was going crazy. Turned out it was the generic. My doc didn’t even know the manufacturer changed. Now I always ask: ‘Who made this?’ and I write it down. My pharmacy hates me. Worth it.
Brett Pouser
February 21, 2026 AT 12:50I’m from the States, but I’ve lived in Ireland and Australia too. Here’s the thing: in places like Ireland, they don’t switch psych meds without a full review. In Australia, pharmacists have to log the manufacturer and notify the prescriber. Here? You get a new pill. You don’t even know. It’s wild. We treat mental health like a commodity. But your brain doesn’t care about insurance networks. It just wants stability. We need to stop pretending cost savings are worth the risk.